Introduction: The role of dendritic cells in the presentation of foreign antigens to induce an adaptive immune response is critical for protection from potentially harmful pathogens. However in the treatment of hemophilia A, the recognition, processing, and presentation of factor VIII (fVIII) by dendritic cells that results in anti-fVIII inhibitors is a devastating complication of modern hemophilia A care. FVIII structure consists of A1, A2, B, A3, C1, and C2 domains. While prior studies have described a role for the C1 and C2 domains in mediating fVIII uptake by dendritic cells, the contribution of the remaining fVIII domains has not been fully explored. In this study we analyzed the full spectrum of fVIII domains on uptake by dendritic cells as a means to better understand the pathogenic innate immune response to fVIII.
Methods: Bone marrow cells were harvested from the femurs and tibias of 8-10 week old E16 hemophilia A knockout mice, stimulated with GM-CSF, and cultured at 37°C for 6-7 days to induce bone marrow derived dendritic cells (BMDCs). BMDCs were incubated with dylight650-conjugated recombinant full length fVIII (DyL650-rfVIII) in combination with a panel of 17 previously characterized anti-fVIII monoclonal antibodies (MAbs) from our repertoire of anti-human murine derived MAbs representing B cell epitopes from all fVIII domains. Internalization of 10 nM fVIII by CD11c+ BMDCs in the presence of molar excess of individual anti-fVIII MAbs (80 nM) following a 30 minute incubation at 37°C was compared to uptake of fVIII alone. FVIII uptake by BMDCs at 4°C was used as a negative control. FVIII internalization was analyzed by flow cytometry and imaged using an Imagestream X Mark II flow cytometer. A supraphysiologic dose of fVIII was utilized in these experiments for optimal visualization and measurement of fVIII internalization by flow cytometry, though similar trends in uptake pattern at more physiologic concentrations of fVIII have also been observed in previous studies performed.
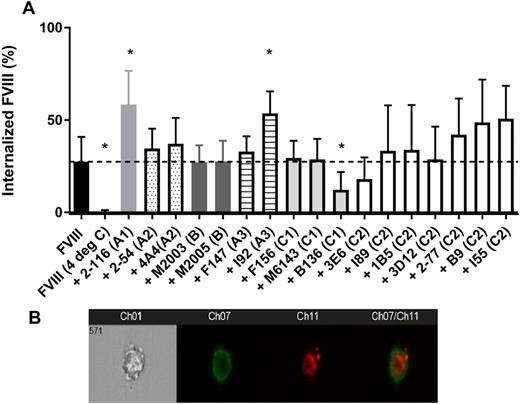
Results: The panel of 17 anti-fVIII MAbs tested included 1 anti-A1, 2 anti-A2, 2 anti-B, 2 anti-A3, 3 anti-C1, and 7 anti-C2 domain MAbs. In this model, the presence of anti-C1 domain and anti-C2 domain MAbs reduced fVIII uptake by BMDCs as shown in prior studies. Anti-C1 domain MAb B136, a group B MAb that binds a critical structural epitope within the 2077-2084 region of the C1 domain, reduced fVIII uptake by 56%. Additionally classical group A anti-C2 domain MAb 3E6 reduced fVIII uptake by 35%. Both MAbs B136 and 3E6 bind to epitopes that are important for fVIII binding to von Willebrand factor and phospholipid surfaces suggesting that these epitopes may play a role in fVIII recognition by dendritic cells, particularly in instances when fVIII is unbound by either ligand in plasma. Interestingly anti-A1 domain MAb 2-116 and anti-A3 domain MAb I92 that binds residues 1818-1916 located at the C terminal end of the A3 domain significantly increased fVIII uptake by BMDCs two-fold (113% and 95% respectively) compared to fVIII alone. These findings indicate that epitopes within the A1 and A3 domains may play a role in limiting fVIII recognition by dendritic cells. All of the anti-A2 and anti-B domain MAbs, 2 of the 3 anti-C1 MAbs, and 5 of the 7 anti-C2 MAbs tested did not alter fVIII uptake. The figure below depicts a bar graph summarizing fVIII internalization by BMDCs with or without the anti-fVIII MAbs tested (figure 1A) and a representative Imagestream image of a dendritic cell (Ch01), CD11c+ surface staining of the dendritic cell (Ch07), DyL650-rfVIII alone without an anti-fVIII MAb (Ch11), and a merged image of CD11c+ dendritic cell and DyL650-rfVIII (Ch07/Ch11) (figure 1B).
Conclusions: The C1 and C2 domains of fVIII primarily mediate fVIII uptake by dendritic cells. However the A1 and A3 domains may play a protective role in antigen uptake and ultimately antigen presentation by dendritic cells. Further investigation into the innate immune response to fVIII in patients with hemophilia A and differences between patients who do and do not develop inhibitors would provide insight into key aspects of the immune response that regulates fVIII tolerance.
Batsuli: Genentech, Octapharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal